Changing the Treatment of NASH
Finding A Solution to Fatty Liver Diseases

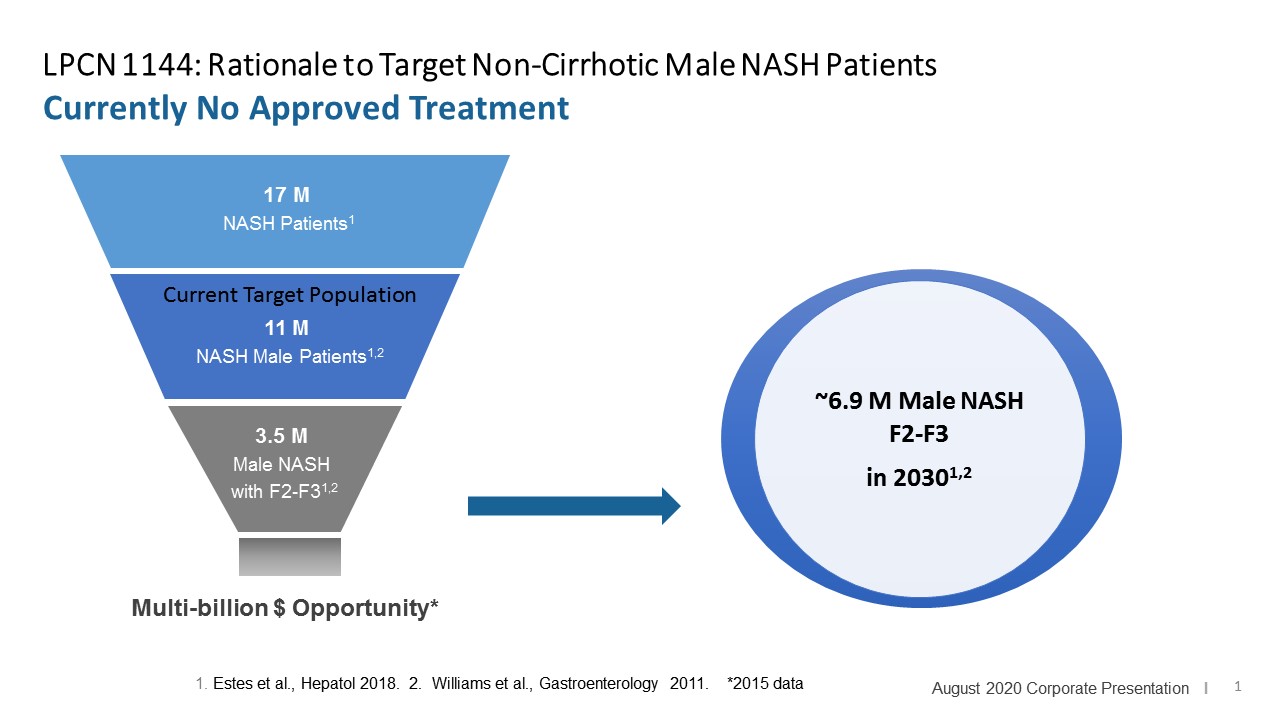
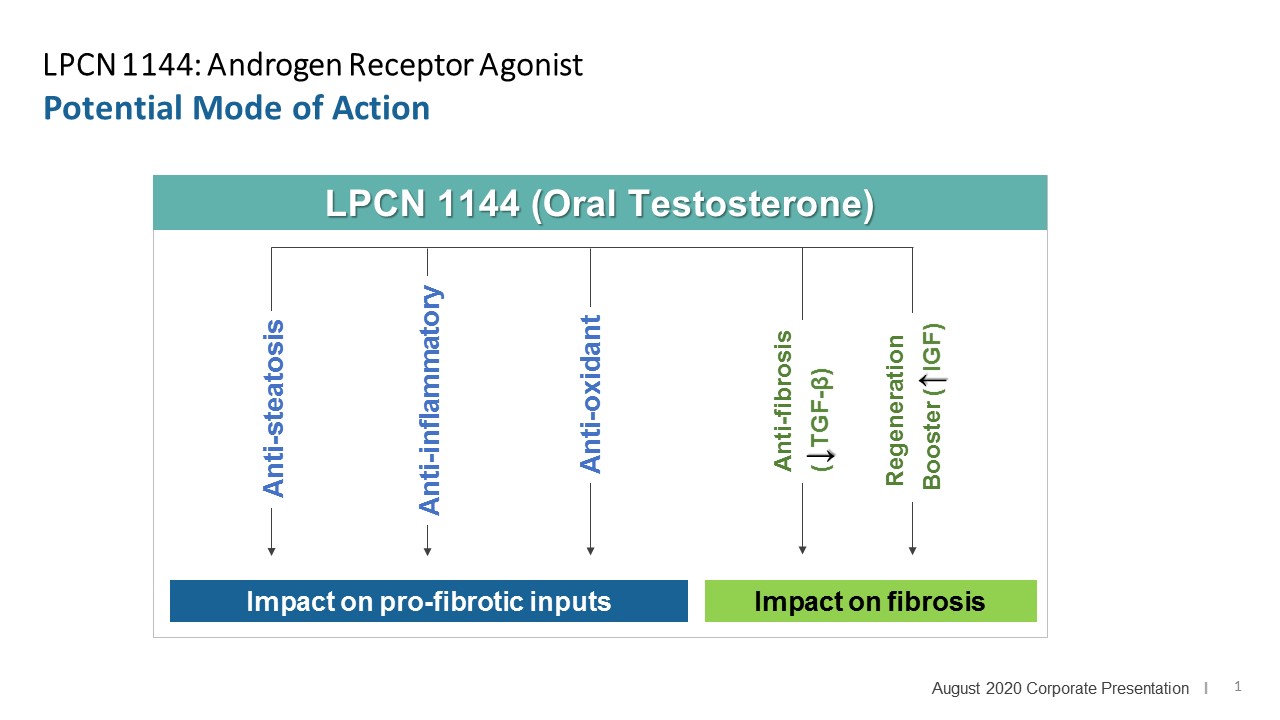
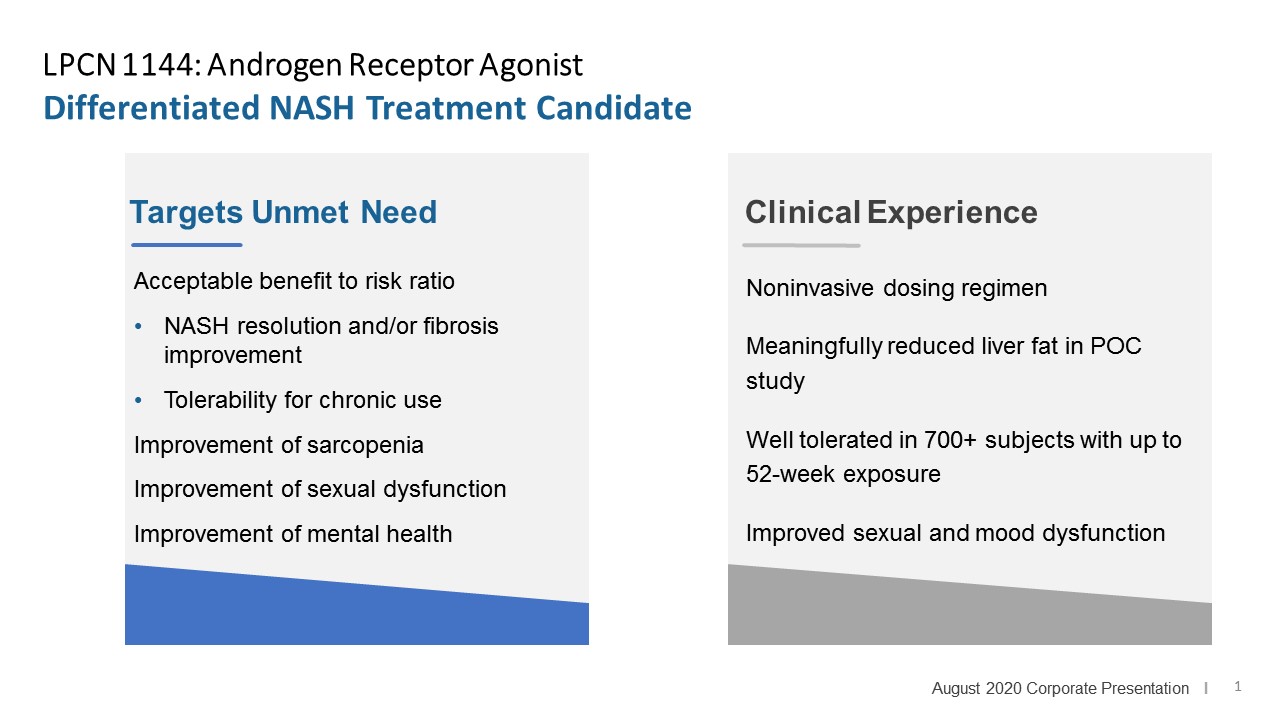
Targeted For Non-Alcoholic Steatohepatitis (NASH)
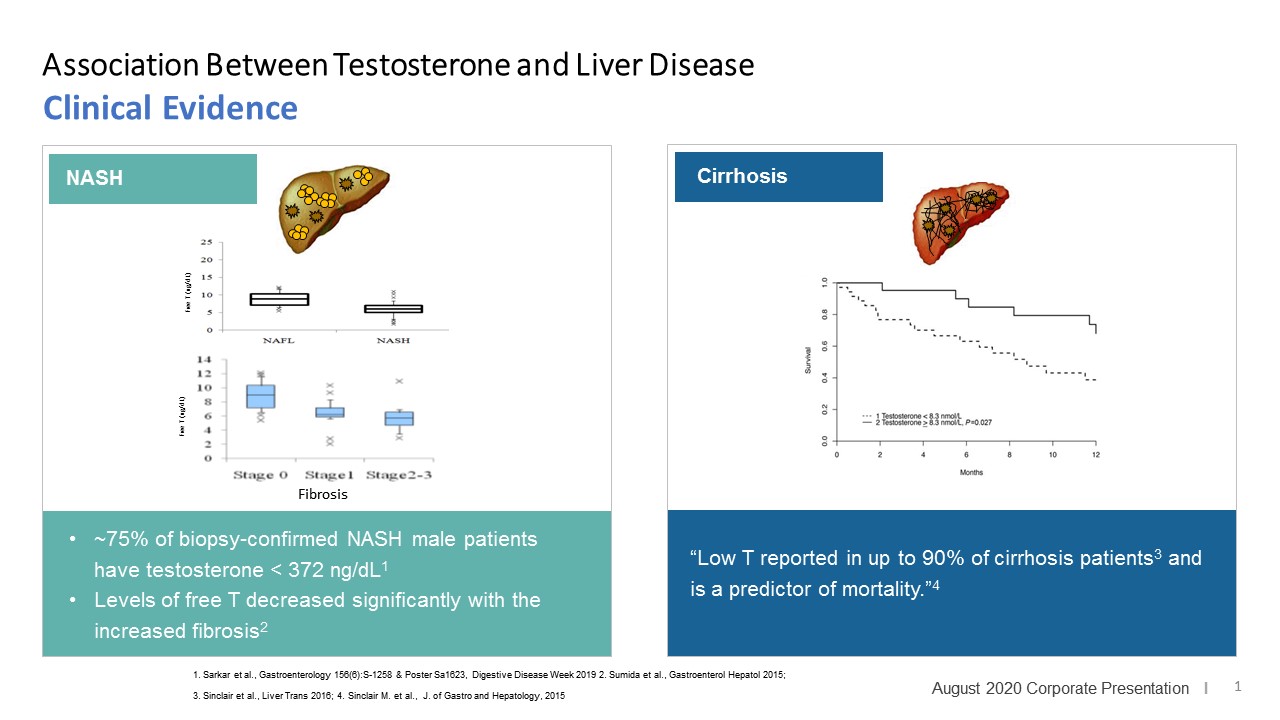
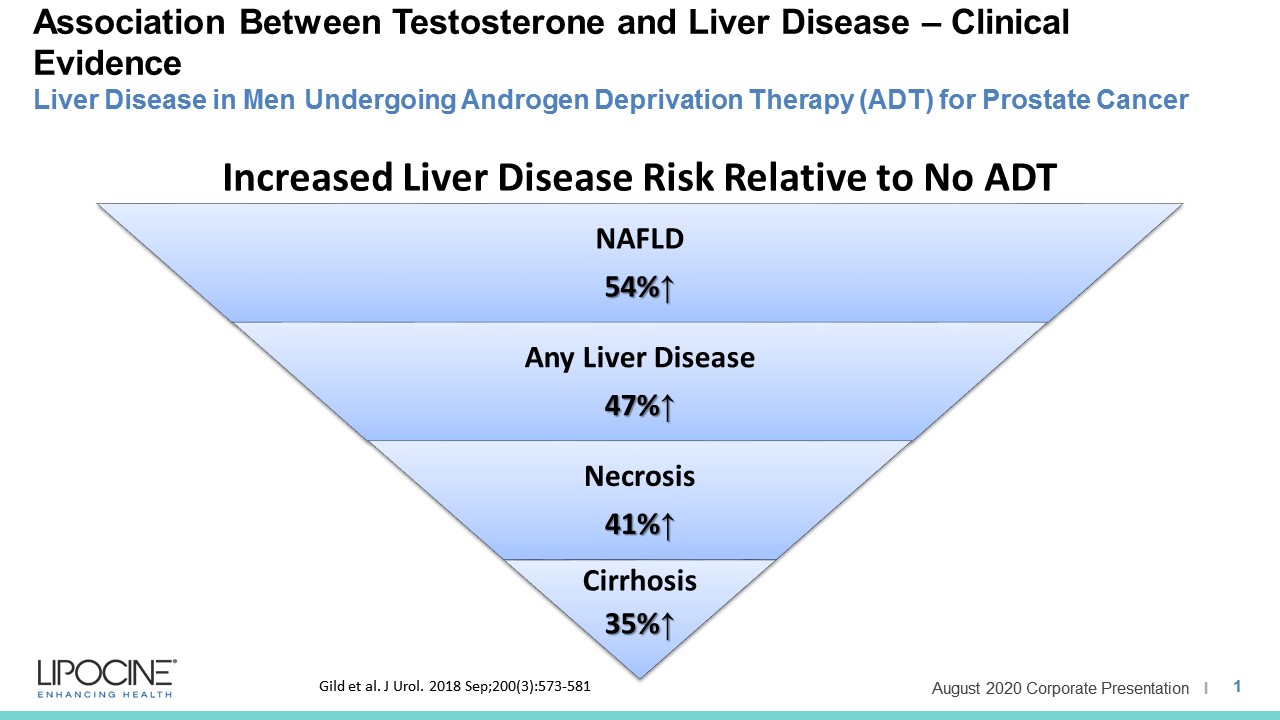
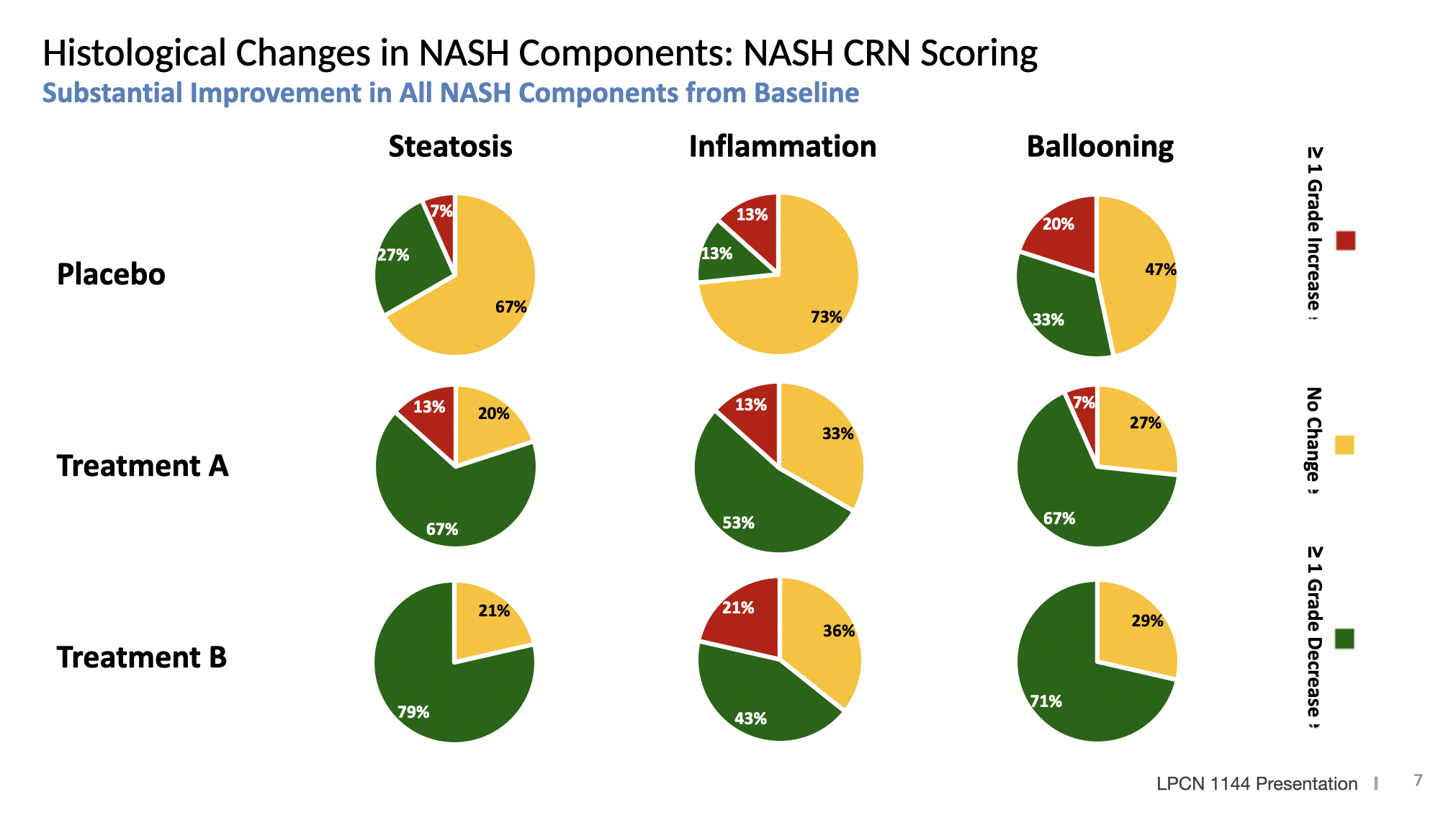
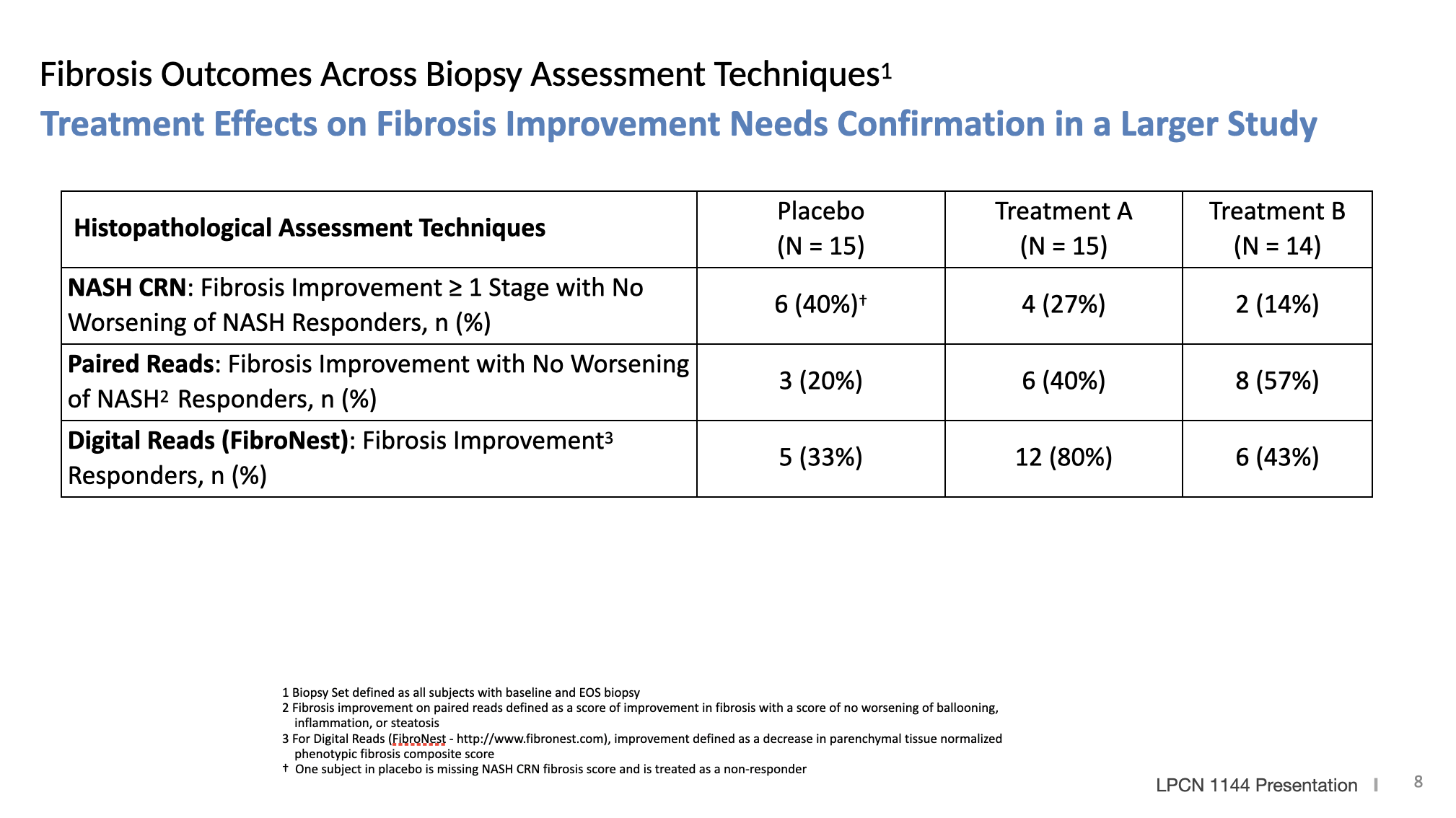
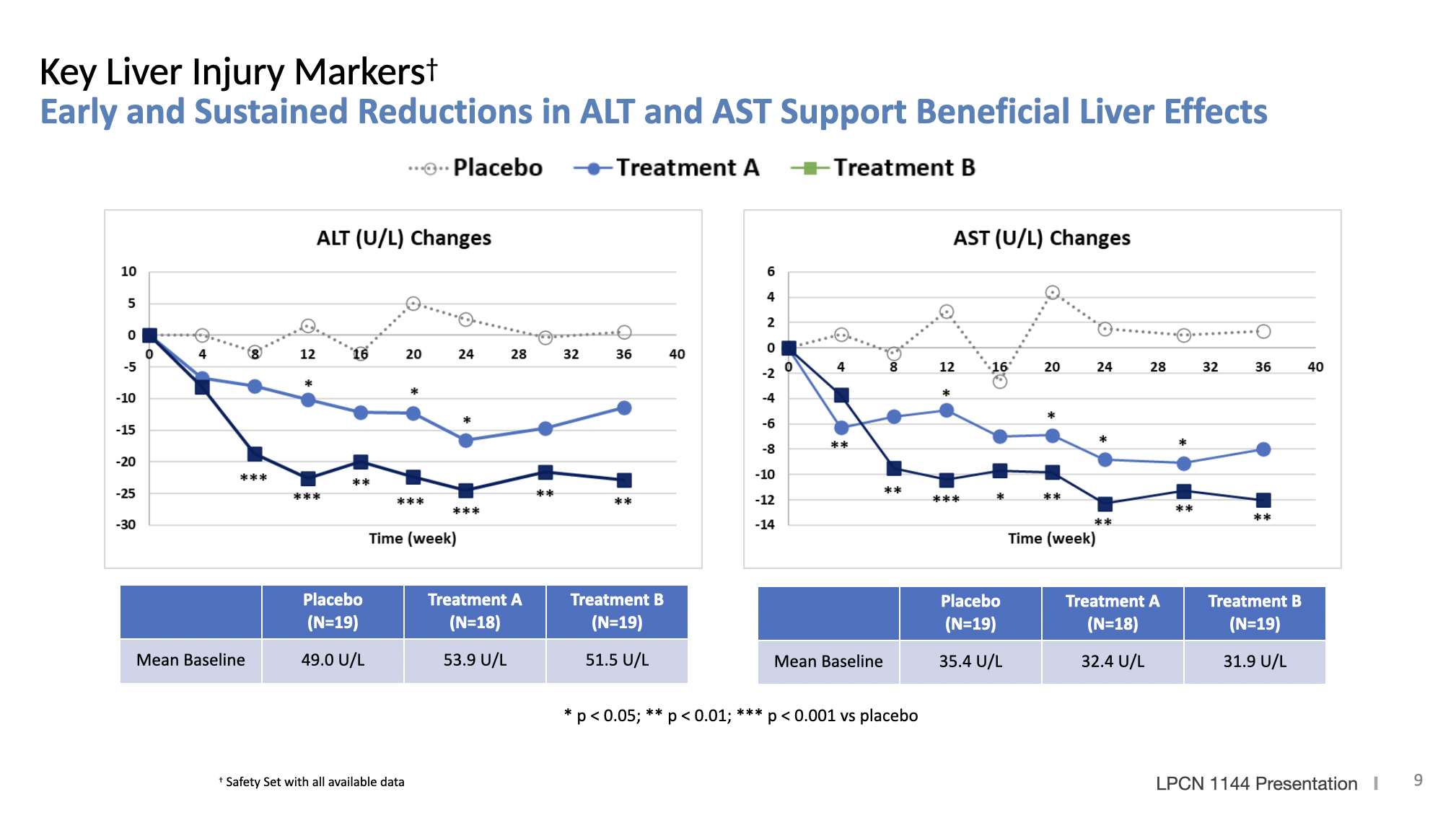
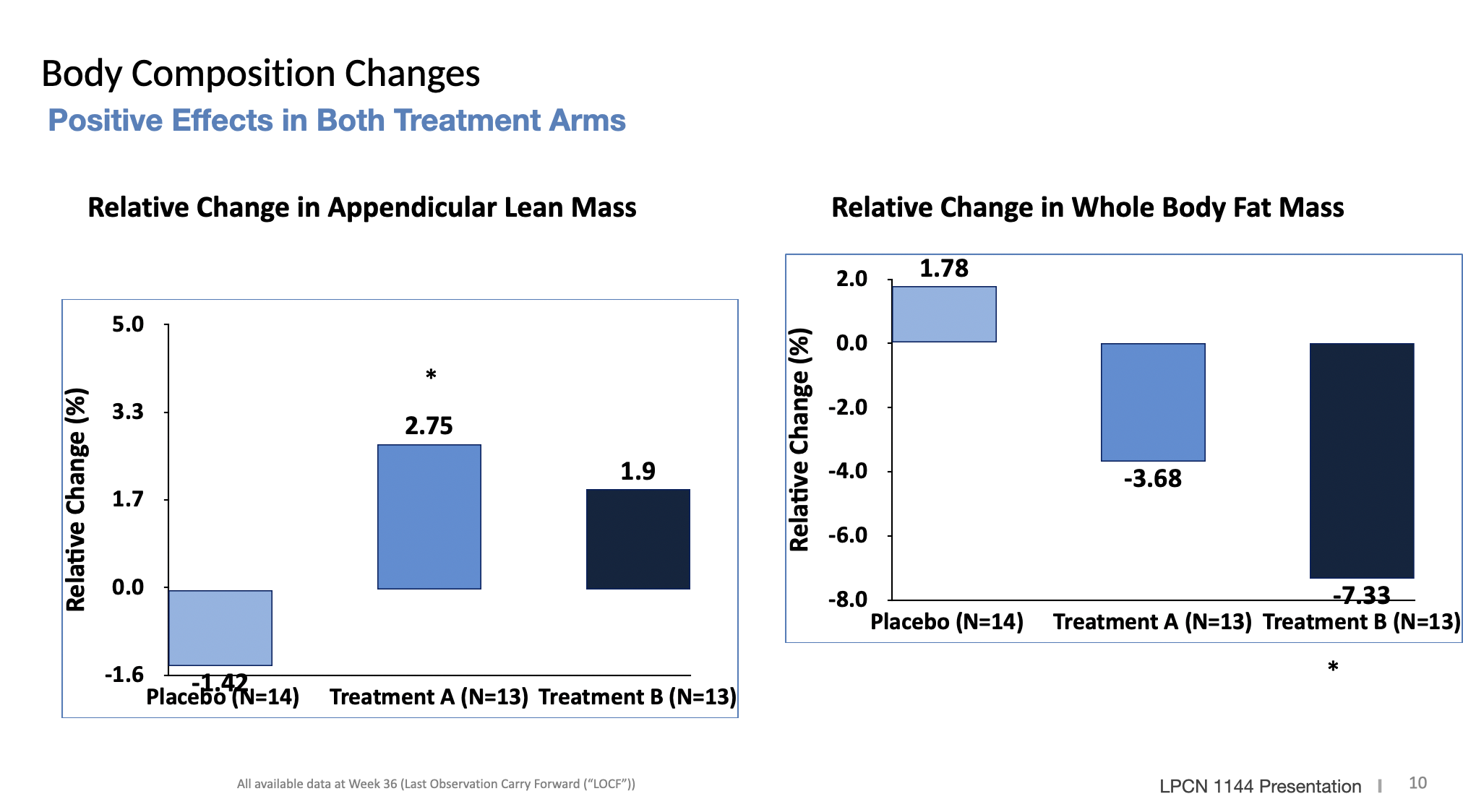
LPCN 1144, an oral prodrug of bioidentical testosterone, is being developed as a treatment for pre-cirrhotic non-alcoholic steatohepatitis ("NASH") and recently completed a Phase 2 paired biopsy NASH confirmed clinical study.
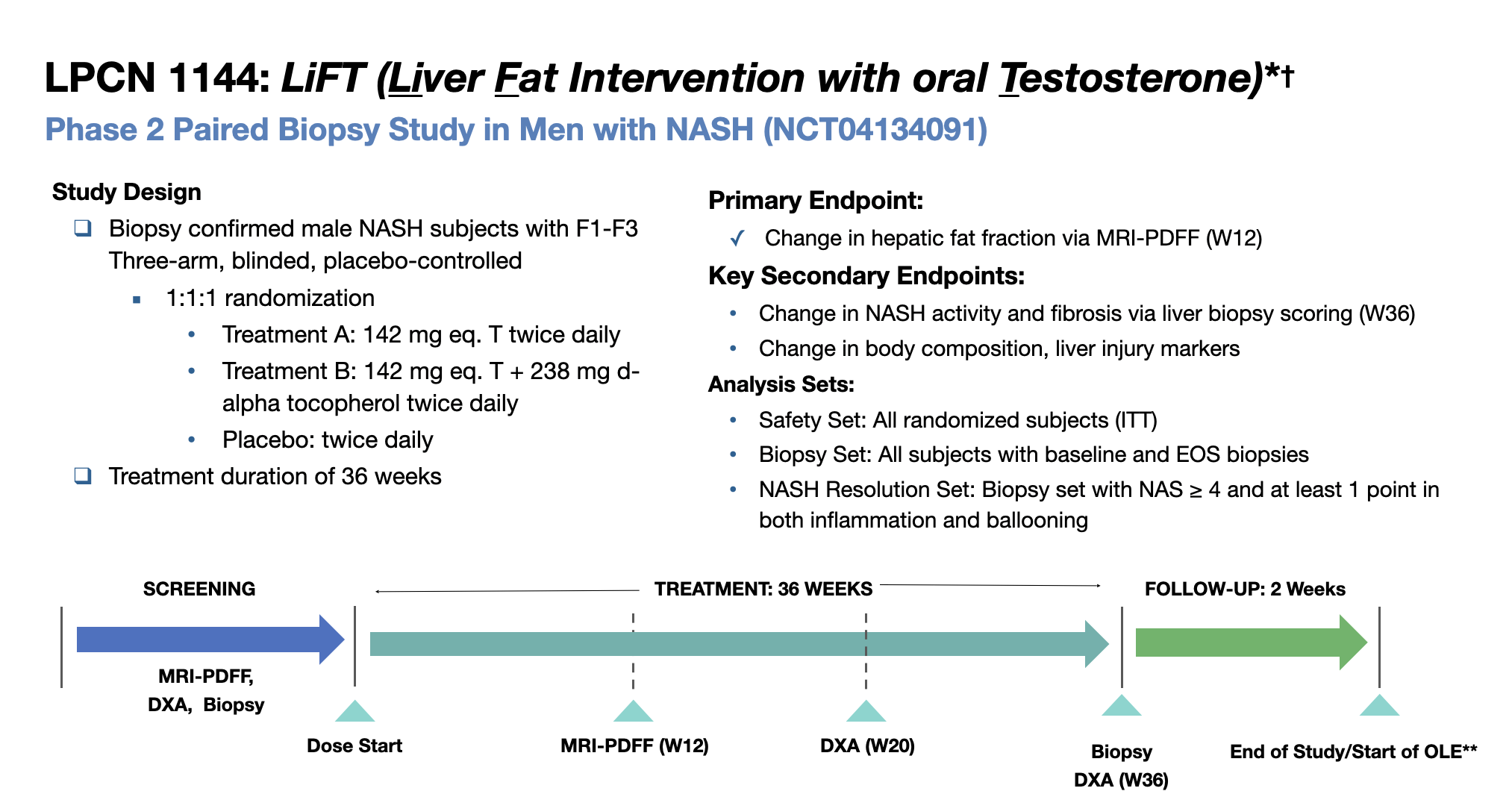
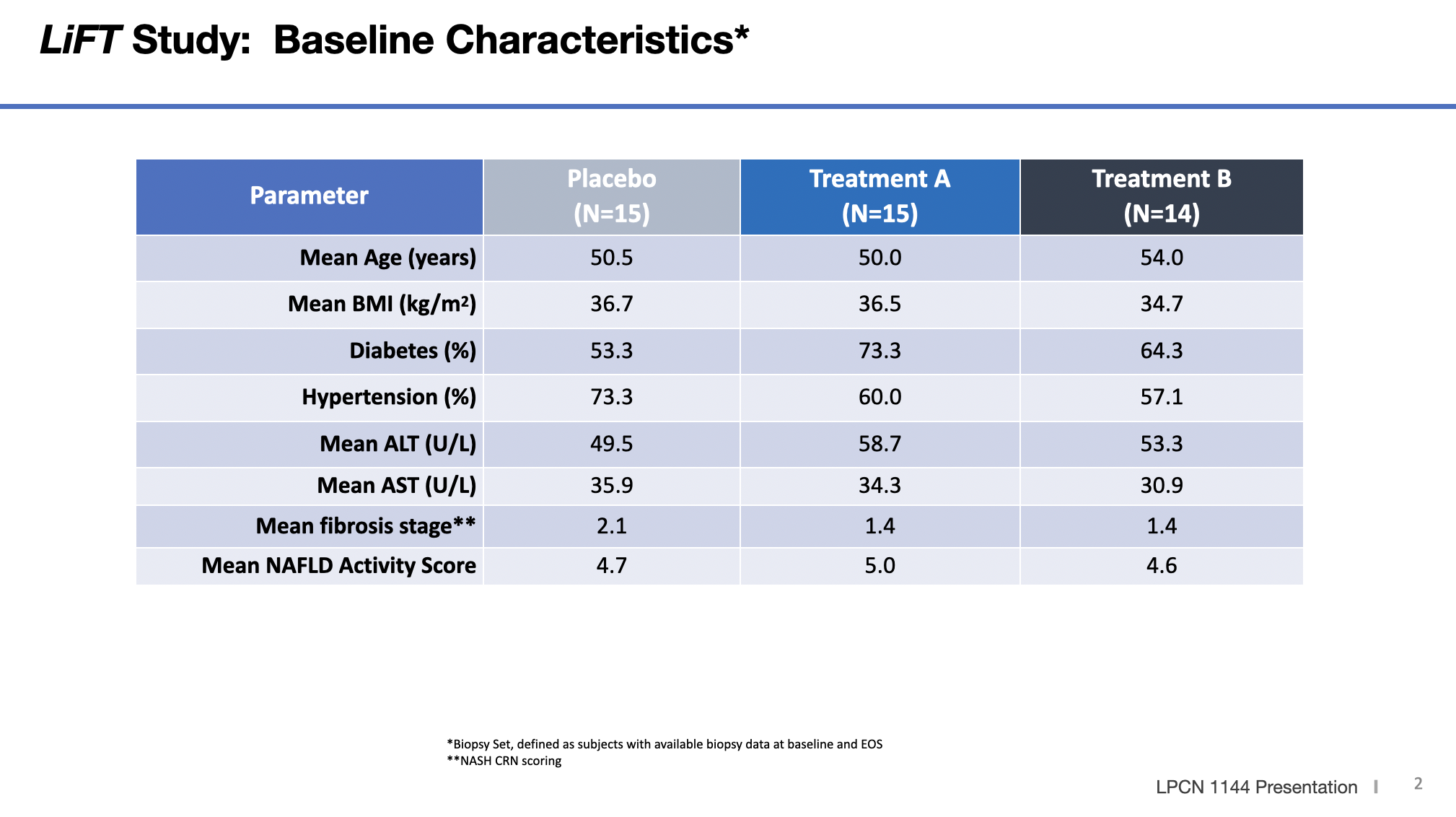
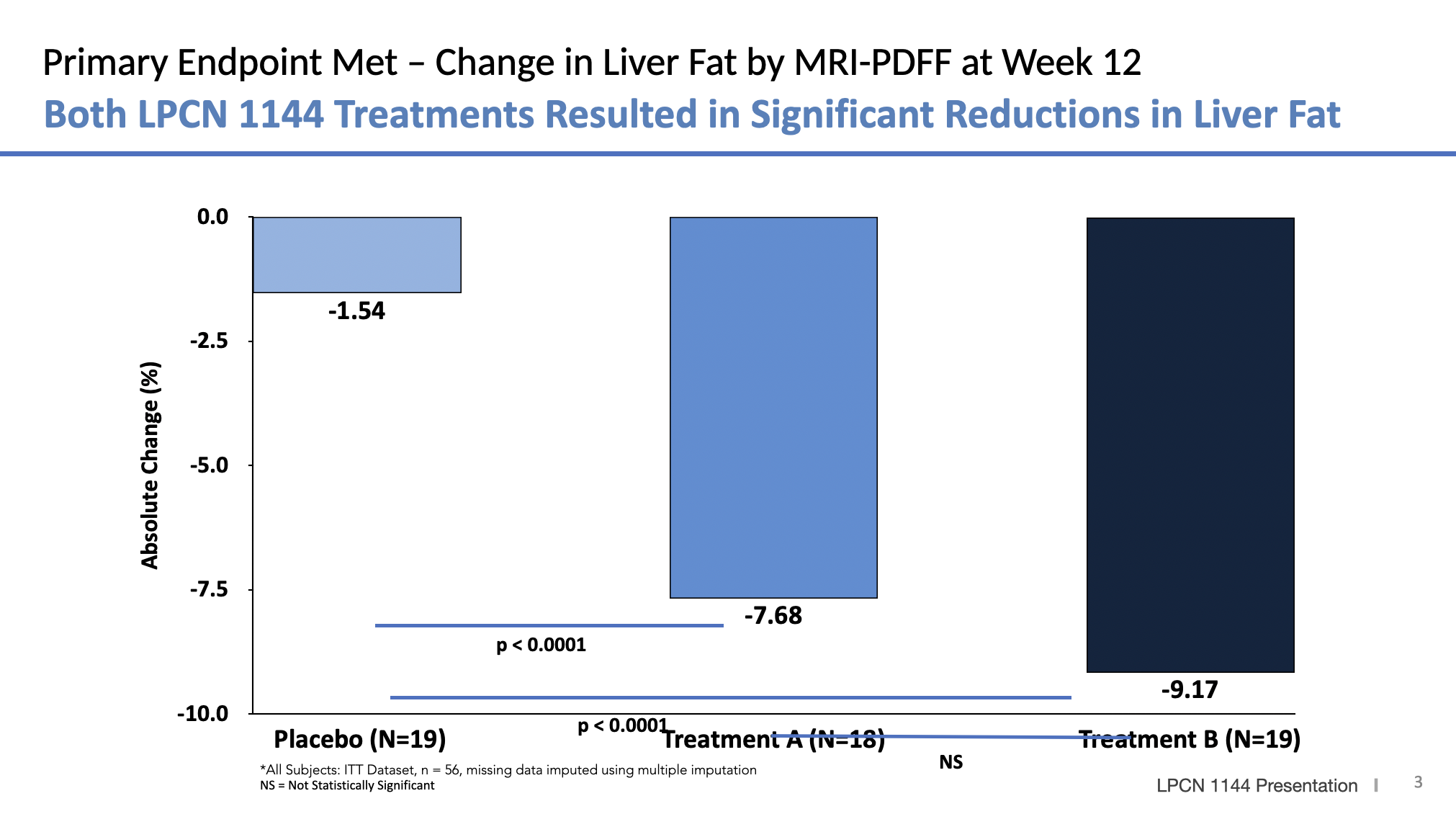
LFS was an open-label, multi-center single-arm 16-week study (N=36) with LPCN 1144 in hypogonadal males
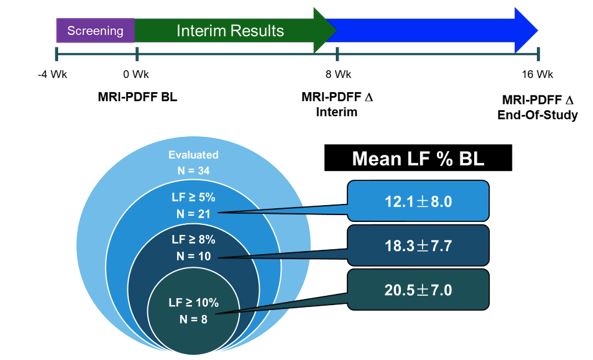
*LF = Liver Fat
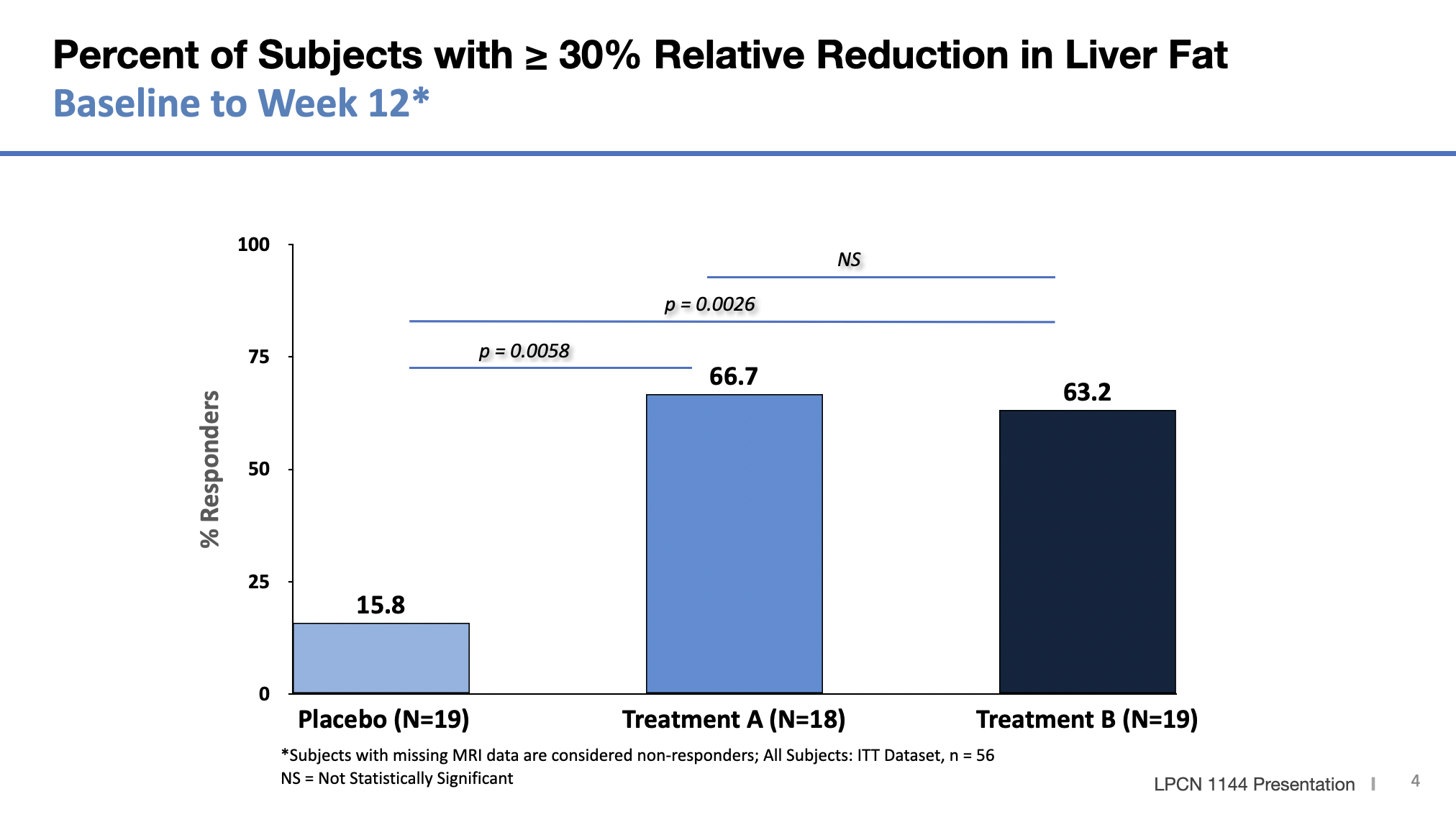
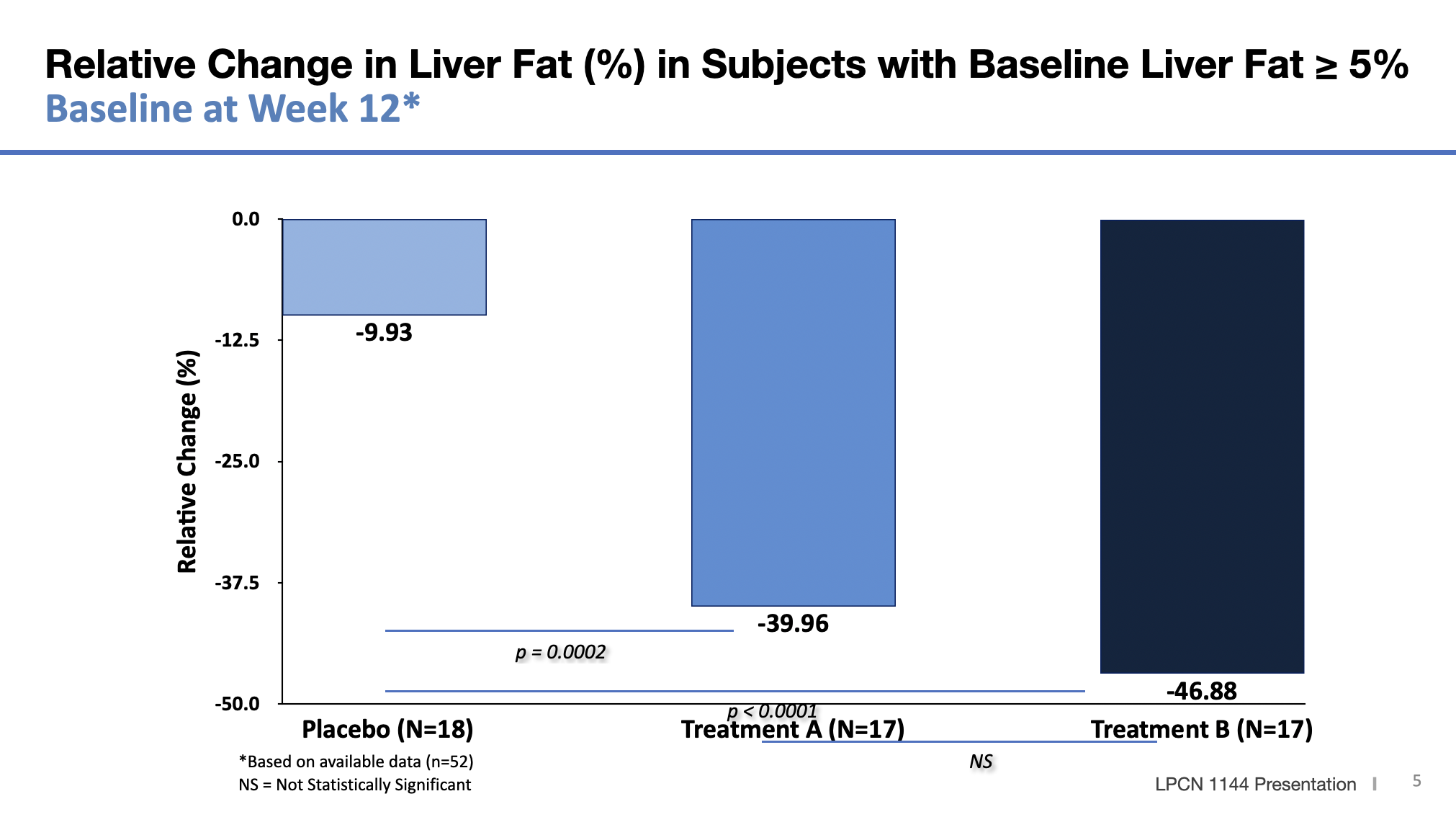
Meaningful Relative Liver Fat % Change and Responder Rate
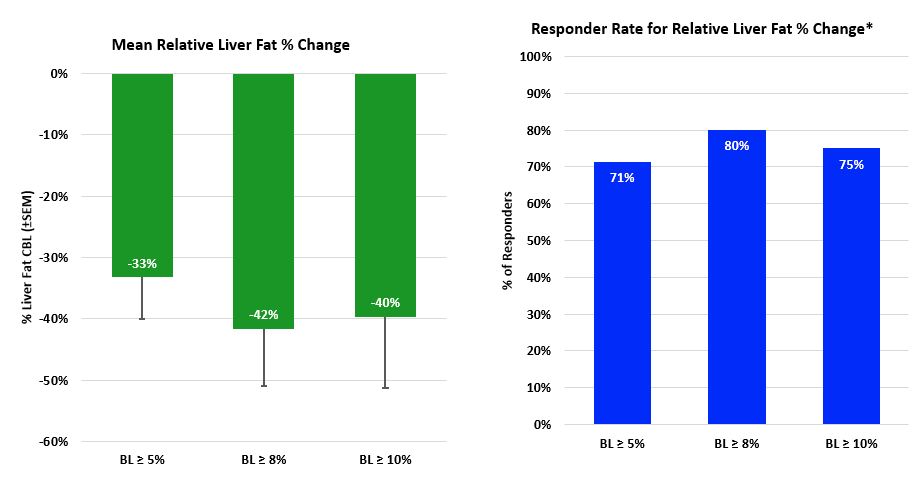
* Responder rate for relative change is % of patients with at least 30% for relative change from baseline.
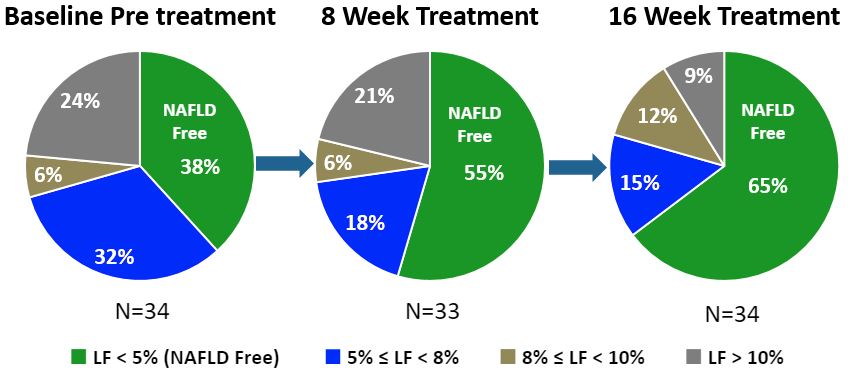
Liver Fat-Based Subject Distribution at Each Visit
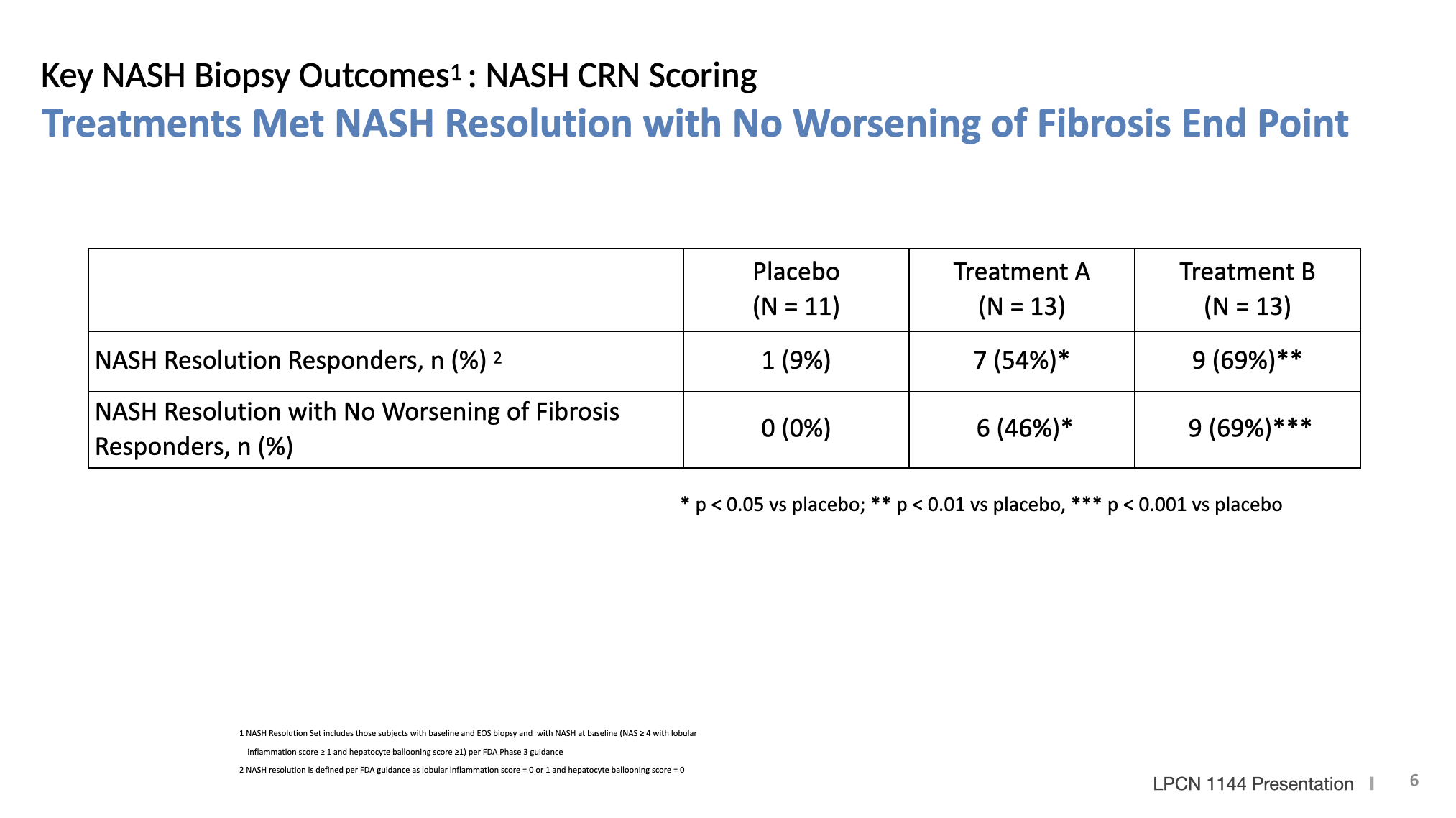
Longer Therapy Improved Proportion of Subjects with Disease Resolution