LPCN 1107
Oral Product Candidate being Developed For The Prevention Of Recurrent Pre-Term Birth

Reducing
Pre-Term Births
LPCN 1107 is an oral product candidate of 17-alpha-hydroxyprogesterone caproate (HPC) under development for the indication of prevention of recurrent preterm birth. LPCN 1107 has the potential to become the first oral HPC product for the prevention of preterm birth in women with a prior history of at least one preterm birth. Potential benefits of our oral product candidate relative to current injectable products include the elimination of pain and site reactions associated with weekly injections, elimination of weekly doctor visits or visits from the nurse, and elimination of interference/disruption of personal, family or professional activities associated with weekly visits.
Market Overview Of Pre-Term Birth
Preterm Birth (PTB) is defined as delivery of less than 37 weeks of gestation. PTB occurs in ~12% of all US births. PTB remains the leading cause of perinatal mortality and morbidity, accounting for as many as 75% of perinatal deaths.
The expense associated with PTB involves not only the immediate cost of the preterm baby being treated in the hospital ICU setting, but includes the long-term treatment costs for disabilities for the life of the child. Current total PTB related economic impact on the US health system far exceeds $26 billion, an estimated cost in 2006.
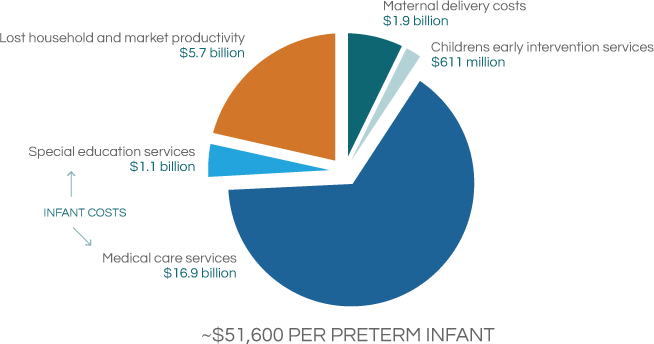
Behrman RE et al. in: Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2006:329-354.
There is a significant unmet need for a ‘patient friendly’ product for the prevention of PTB. The only FDA approved the product for the prevention of PTB must be given by either a sub-cutaneous or intra-muscular injection each week for a total of 18-22 injections.
