Proprietary Solubilization Technology
ENTAILS REPOSITIONING OF ESTABLISHED DRUGS WITH SIGNIFICANTLY IMPROVED PATIENT COMPLIANCE.
Lipocine pipeline products are based on its proprietary solubilization technology for effective oral delivery of water insoluble drugs to improve patient compliance.
LPCN 1144 is an oral prodrug of bioidentical testosterone that is being developed as a treatment of non-alcoholic steatohepatitis (NASH) and recently completed the Phase 2 LiFT ("Liver Fat intervention with oral Testosterone") clinical studies
TLANDO®
An FDA approved oral TRT option that does not require dose titration. Lipocine licensed the exclusive U.S. rights for TLANDO® to its commercialization partner, Verity Pharmaceuticals.
LPCN 1111 is a novel next-generation oral prodrug of testosterone with potential for once-daily oral dosing that has completed Phase 2 testing in hypogonadal men. Good dose-response relationship was observed over the tested dose range.
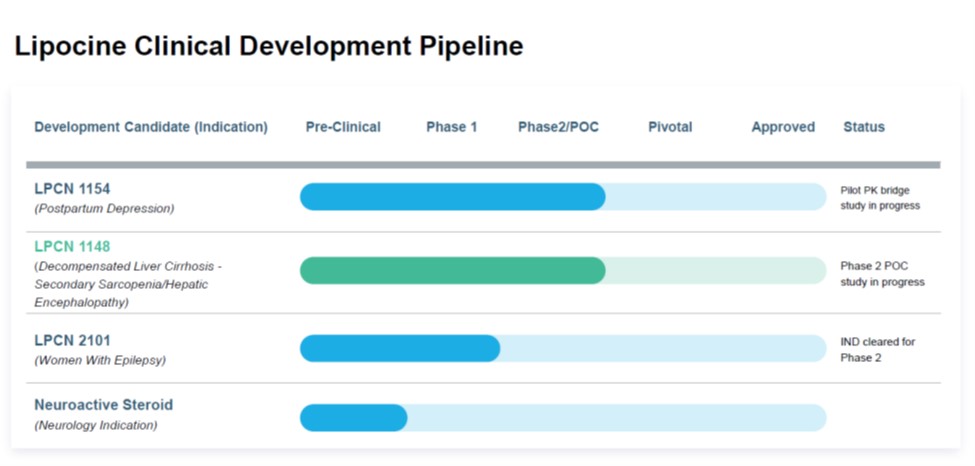
LPCN 1148 is an oral prodrug of bioidentical testosterone being developed for the management of symptoms associated with cirrhosis.
LPCN 1107 is targeted to be the first oral hydroxyprogesterone caproate product candidate indicated for the prevention of recurrent preterm birth. LPCN 1107 has been granted orphan drug designation by the FDA and has completed an end of Phase 2 meeting with the FDA.
LPCN1154 Is an oral neurosteroid being developed for the treatment of postpartum depression.
